Describe the Structure of the Nuclear Atom
Carbon is described as The chemical element of atomic number 6 a nonmetal that has two main forms diamond and graphite occurs in impure form in charcoal soot and coal and is present in all organic compounds What system is. The number of protons is call the Atomic Number.

Atom Rutherford S Nuclear Model Britannica
An atom therefore cannot be described using the modern atomic model without knowing which element the atom is made of as the element determines.

. The shells of an atom are numbered 12 3 and so on starting from the one closest to the nucleus. Learn vocabulary terms and more with flashcards games and other study tools. Carbon Atom What is it.
42 structure of the nuclear atom answers. Atomic of or relating to or comprising atoms. Learn vocabulary terms and more with flashcards games and other study tools.
The proton charge is exactly the same as the electron charge but of opposite signThis means that in any electrically neutral atom the number of protons in the nucleus often referred to as the nuclear charge is balanced by the same. Each shell can occupy a certain number of. An atom consists of negatively charged electrons arranged in shell with a positively charged nucleus.
This model of the atom turned out to be short-lived however due to the work of Ernest Rutherford 18711937. The nucleus contains protons and neutrons. Thomson described the structure of the atom as a positively charged sphere into which negatively charged electrons were entrenched on the basis of his experiment.
The basic structure of an atom is defined as the component-level of atomic structure of an atom. The Rutherford atomic model is known as the nuclear atom. 42 Structure of the Nuclear Atom.
The structure of an atom is in the middle of an atom has the neutron nucleus which has no charge around the neutron is the protons which has a positive charge and the ones that orbit around the atom is the electrons which has a negative charge in it. Atomic number and mass number. Rutherfords gold foil experiment provided evidence for the atomic nucleus a small dense core of the atom which contains the positive charge and most of the mass.
The Nuclear Atom Almost all of the mass of an atom is contained within a tiny and therefore extremely dense nucleus which carries a. Electrons revolve around the nucleus in a circular orbit similar to the way planets orbit the sun. An atom has a central nucleus.
The electrons are distributed around the nucleus and occupy almost all the volume of the atom. In the nuclear atom the protons and neutrons are located in the positively charged nucleus. In the nuclear atom the protons and neutrons are located in the positively charged nucleus.
Number of neutrons 9 4 5 b. Neutron elec-tron and proton. Electrons are arranged around the nucleus in the shells of an atom.
Describe the modern model of the atom. Protons are the carriers of positive electric charge in the nucleus. The nucleus is itself composed of two kinds of particles.
The popular name of Thomsons model is plum pudding model because it can be seen as a plum pudding dish where the pudding means the positively charged atom and the plum pieces stand for the. The nucleus is surrounded by a cloud of negatively charged electrons. 42 42 Structure of the Nuclear Atom What evidence from Rutherfords Gold-Foil experiment disproves JJ.
The central core of an atom which is composed of protons and neutrons c. 42 The Atomic Nucleus JJ. The radius of an atom is about 01 nm 1 10-10 m the.
Atomic structure is spherical. Almost all of the volume of an atom consists of empty space in which electrons the fundamental carriers of negative. The nuclear model of the atom is one in which the nucleus is composed of protons and neutrons while electrons are distributed throughout the rest of the space.
The Electronic Structure of an Atom. This is surrounded by electrons arranged in shells. Guide for Reading Build Vocabulary Word Parts Have students think of words that start with the same word parts as the three subatomic particles described in this section.
Atomic hydrogen The basic unit of a chemical element Such particles as a source of nuclear energy. The proton charge is exactly the same as the electron charge but of opposite sign. Thompson and others supposed the atom was filled with positively charged material and the electrons were evenly distributed throughout.
The structure of the nuclear atom with a central nucleus and surrounding electrons. 422 Describe the structure of atoms according to the Ruther-ford atomic model. Section 42 the structure of an atom pages 108-112 answer key By the end of this section you will be able to.
Protons are the carriers of positive electric charge in the nucleus. Describe the structure of an atom. Bohrs Model Of The Atom.
Start studying Structure of the Nuclear Atom. Protons have a relative charge of 1 and the. The atom consists of a small but massive nucleus surrounded by a cloud of rapidly moving electronsThe nucleus is composed of protons and neutronsTypical nuclear radii are of the order 10 14 m.
The English chemist Sir Joseph John Thomson put forth his model describing the atomic structure in the early 1900s. Structure of the Nuclear Atom. Big Ideas in sales.
The central region of an atom has a very small positively-charged nucleus which contains almost all the mass of the atom. Extension Activity 1 - Construct a 3D Model of an Atom. Precisely speaking an atom consists of three major subatomic particles which are protons neutrons and electrons.
The nucleus is tiny compared to the atom as a whole. Describe and model the structure of the atom in terms of. The nucleus is itself composed of two kinds of particles.
Rutherfords Structure of Atom The nucleus is at the center of an atom where most of the charge and mass are concentrated. How can you describe the structure of the nuclear atom. Assuming spherical shape nuclear radii can be calculated according to the following formula.

Atomic Structure Parts Of The Atom Bundle Middle School Lessons Education Lesson Plans Education

Elektrichestvo Plum Pudding Model Atomic Theory Atom Model

Pengertian Partikel Penyusun Atom Menurut Para Ahli Http Www Gurupendidikan Com Pengertian Partikel Penyusu Chemical Energy Nuclear Energy Atomic Structure

Nucleus Definition Structure Function Cellular Vs Atomic Nuclei Nuclear Membrane Cell Organelles Quotes For Book Lovers

Atom Gifs Atomic Structure Atom Structure Definition
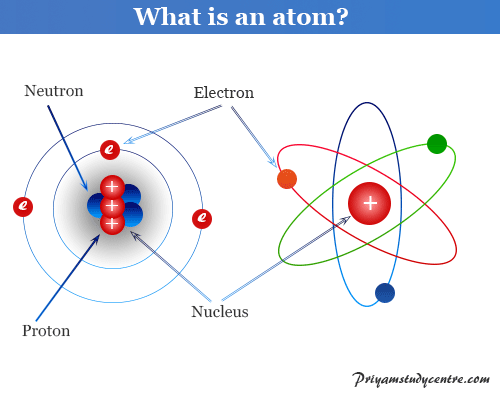
Atom Structure Definition Theory Examples Diagram

Atom Gifs Atomic Structure Atom Structure Definition

Atomic Structure Vocabulary Card Sort In 2022 Teaching Chemistry Chemistry Classroom Chemistry Lessons

The Nuclear Atom Atoms Differ From One Another By Their Number Of 1 Protons 2 Neutrons 3 Electrons Electrons Have A Negative Charge The Mass Of An Electron Ppt Download

Diagram Of An Oxygen Atom Atom Project Atom Model Project School Science Projects

What Is Electricity What Is Electricity Electronics Projects Diy Electricity

Chemistry Atom Structure Animations At Best Animations Imagenes De Dj Ilusiones Opticas Geometria

Atomic Structure Wyzant Resources Nuclear Energy Chemical Energy Atomic Structure

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Atom Project

2 1 2 2 Atomic Structure Sl Youtube

15 P Phosphorus Electron Shell Structure Schoolmykids Element Chemistry Periodic Table Of The Elements Aluminum Element

Atomic Structure Nucleus Proton Neutron Electron Mass Charge Isotopes Electron Arrangement Rutherford Bohr Model Of Atom Allotropes History Of Atomic Structure Model Development Ionisation Ions Gcse Chemistry Revision Notes Quizzes Ks4 Science

Potassium Electron Configuration Potassium Atom Potassium Electron Configuration

Image Of An Atom Google Search Atomic Theory Bohr Model Dalton Atomic Model
Comments
Post a Comment